Alongside Mr Nicol's Videos I found this on very helpful
http://www.youtube.com/watch?feature=player_embedded&v=HACQtNdQKj8
Tuesday 15 October 2013
Monday 14 October 2013
Thursday 19 September 2013
Wednesday 18 September 2013
Oxidation-Reduction Introduction
Here is a quick overview of the content of the Oxidation-Reduction topic that we will be working on in Term Four.
Monday 16 September 2013
Saturday 14 September 2013
Friday 16 August 2013
Lucas Reagent
Lucas Reagent provides a high concentration of chloride ions. These ions can replace the hydroxy (-OH) groups of secondary and tertiary alcohols.
Wednesday 14 August 2013
Tuesday 13 August 2013
Alcohols Introduction
The video below outlines the functional group, general formula, physical properties and classification of alcohols.
Monday 12 August 2013
Friday 9 August 2013
Wednesday 7 August 2013
Tuesday 6 August 2013
Monday 5 August 2013
Tuesday 9 July 2013
Acidic/Alkaline Salts
Some salts are not truly neutral, despite being made in a neutralisation reaction (acid + base). How can we determine whether a salt is acidic or alkaline? How can we justify this?
Friday 5 July 2013
Friday 28 June 2013
Dynamic Equilibrium and le Chatelier's Principle
A dynamic equilibrium is a reaction which is reversible and the rate of reaction in the forward direction is the same as the rate of reaction in the reverse direction.
le Chatelier's Principle says that this equilibrium will shift (in the forward or reverse direction) to overcome any change made to the system. For example, if more reagent is added, the equilibrium shifts in the forward direction to decrease this amount of reagent. As a consequence, more product is made. The "aim" is to re-establish dynamic equilibrium.
le Chatelier's Principle says that this equilibrium will shift (in the forward or reverse direction) to overcome any change made to the system. For example, if more reagent is added, the equilibrium shifts in the forward direction to decrease this amount of reagent. As a consequence, more product is made. The "aim" is to re-establish dynamic equilibrium.
Tuesday 11 June 2013
Bond Enthalpies
pp143-144 Beginning Chemistry
This is also a pretty good website which could help: http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm
Monday 10 June 2013
Friday 7 June 2013
Endothermic vs. Exothermic
Today, we did four simple experiments to see that some reactions absorb heat energy (feel cold) while others release heat energy (feel warm/hot).
- NaOH + water = increase in temperature (exothermic)
- Ammonium chloride + water = decrease in temperature (endothermic)
- HCl + NaOH solutions = increase in temperature
- Ammonium thiocyanate + barium hydroxide = decrease in temperature
Friday 31 May 2013
Wednesday 29 May 2013
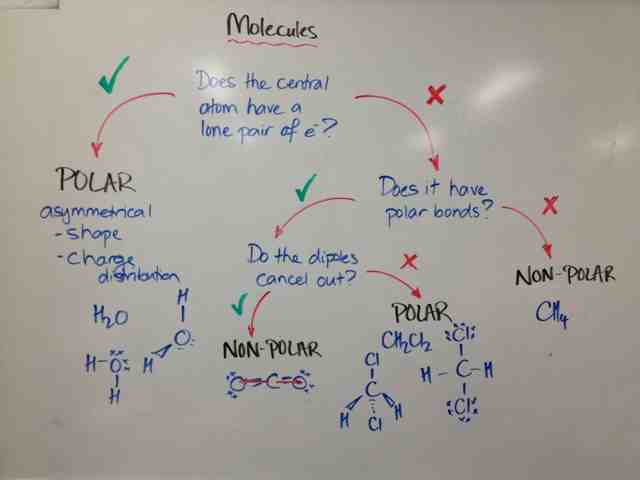
Shapes of Molecules
The shapes of molecules are the last clue for helping determine molecular polarity:
Tuesday 28 May 2013
Lewis Diagrams
Lewis Diagrams and Structures are very useful for helping us predict molecular polarity.
Monday 27 May 2013
Wednesday 22 May 2013
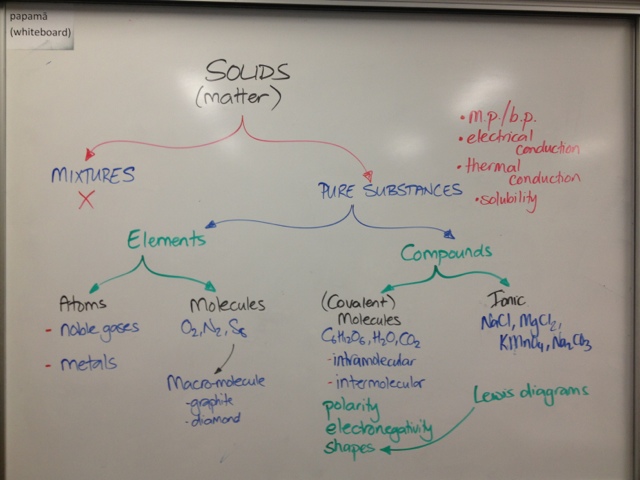
Substance Type Overview
It is very important to understand that "Matt" is not an attractive force, so please do not use it as an answer in assessments.
Tuesday 21 May 2013
Chart - Type of Solid, Type of Particle, Attractive Forces Between Particles
http://chemicalminds.wikispaces.com/file/view/solidsANSWERS.pdf
This is a fully answered chart for multiple types of solids, same type of question from one of the question sheets from today.
This is a fully answered chart for multiple types of solids, same type of question from one of the question sheets from today.
Monday 20 May 2013
Molecular Solids
 Molecular Solids have some characteristic properties:
Molecular Solids have some characteristic properties:- low m.p./b.p.
- electrical and thermal insulators
- often brittle
These properties need to be explained in terms of the forces between the molecules (particles). These forces are called van der Waal's forces. Before we can understand these, we need to understand molecular polarity, which requires us to understand intra-molecular bonding (covalent), shapes and electron cloud size.
Tuesday 14 May 2013
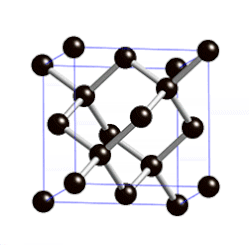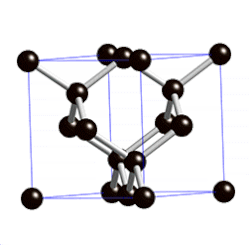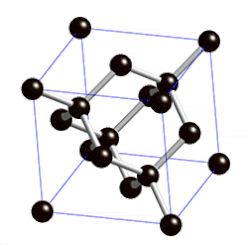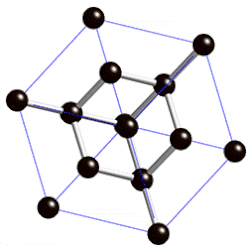
Covalent Networks
Monday 13 May 2013
Metallic Solids
Most elements are metals. Metals have some characteristic properties which can be explained by the metallic bond and the structure of metals in their solid state:
- Electrical Conductivity
- Thermal Conductivity
- Malleability/Ductility
- Relatively High m.p (except Hg)
Wednesday 8 May 2013
Thursday 11 April 2013
Structure and Bonding Introduction
Next term, we will be starting our next topic. Structure and Bonding is part of a bigger topic including Enthalpy Changes (Thermochemistry) and is assessed by AS91164.
Here is a brief overview of what we will be covering in the Structure and Bonding section:
Thank you to Macca for the camera work.
Here is a brief overview of what we will be covering in the Structure and Bonding section:
Thank you to Macca for the camera work.
Wednesday 10 April 2013
Wednesday 27 March 2013
Wednesday 20 March 2013
Tuesday 19 March 2013
Standard Solutions
How to make a standard solution and calculate its precise concentration:
Thank you to Po for the camera work.
Thank you to Po for the camera work.
Monday 18 March 2013
Thursday 14 March 2013
Water of Crystallisation
Many ionic crystals have water molecules withing their structure. We call these hydrated salts. We can drive the water off by heating the salts strongly, creating anhydrous salts (salts with no water inside their crystal structure).
This can be easily seen in substances such as copper (II) sulfate:
However, not all hydrated salts will change colour when dehydrated. Instead, we record the mass of the hydrated salt, then heat it strongly, then record the mass again. If the mass has changed, we can infer that water of crystallisation has been removed by the heating.
The change in mass is the water removed. As we know the molar mass of water (18.0 g mol-1), we can then calculate the amount of water removed. Once we calculate the amount of the anhydrous salt we have left, we can calculate the formula of the hydrated salt:
This can be easily seen in substances such as copper (II) sulfate:
 |
| Hydrated Copper(II) Sulfate is a blue crystal. |
 |
| A crucible is placed on a pipe-clay triangle to heat the hydrated copper(II) sulfate strongly. |
 |
| Anhydrous copper (II) sulfate is a finer powder and is white (left). |
However, not all hydrated salts will change colour when dehydrated. Instead, we record the mass of the hydrated salt, then heat it strongly, then record the mass again. If the mass has changed, we can infer that water of crystallisation has been removed by the heating.
The change in mass is the water removed. As we know the molar mass of water (18.0 g mol-1), we can then calculate the amount of water removed. Once we calculate the amount of the anhydrous salt we have left, we can calculate the formula of the hydrated salt:
EXAMPLE
5.96g of hydrated BaCl2 was heated strongly for 5 minutes, weighed (5.08g), then heated again for another 2 minutes and reweighed (5.08g).
Monday 11 March 2013
Friday 8 March 2013
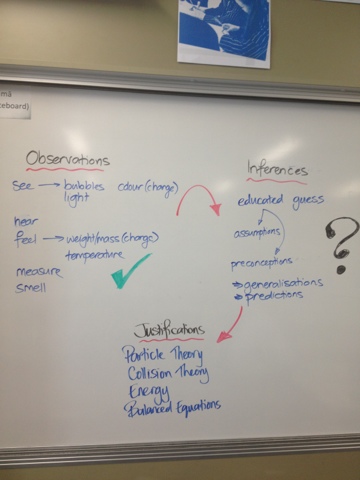
Quantitative Analysis
Today, the class wanted an overview of the topic and to see how the different concepts were linked. Here are the video of this overview and a close-up of the diagram drawn on the whiteboard:
The video is is a bit rough as this was an impromptu teaching moment...
The video is is a bit rough as this was an impromptu teaching moment...
Wednesday 27 February 2013
Monday 18 February 2013
Identifying Cations
This link should take you to the youtube video of Mr Nicoll teaching us about Identifying Cations
Friday 15 February 2013
Tuesday 12 February 2013
What we need to know from the Periodic table
This video was from last week when Mr. Niccol was showing us what we do and don't need from the periodic table. He explains how the columns are related to the charge of each ion. He goes over the most common ones of which we need to know e.g. Na, H, and Al. But also some awkward ones in the middle e.g. Pb, Ag.
Tuesday 5 February 2013
Subscribe to:
Posts (Atom)